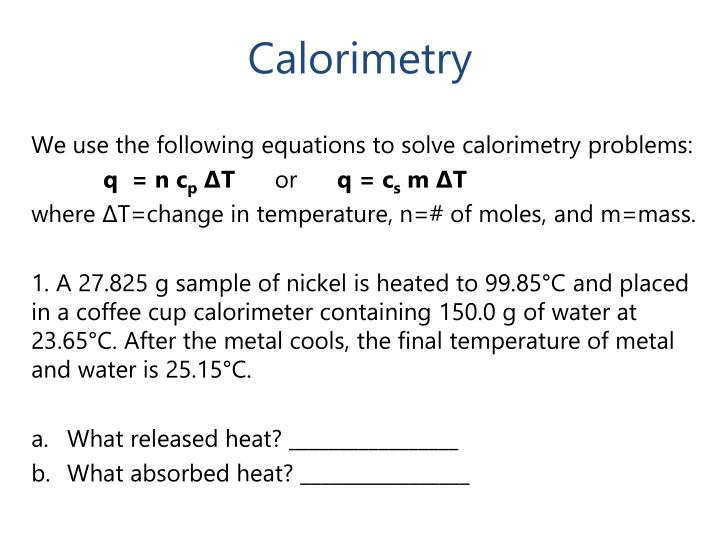
Formula For Calorimeter In Physics . The symbol c stands for the. The formula q = ccal × δt is essential for calorimetry experiments using a calorimeter. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. A physics class has been assigned the task to determine an. Where, solved examples for calorimetry formula. Qsub = m × c × δt. It's not easy to measure the heat transfer directly. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or.
from www.slideserve.com
Qsub = m × c × δt. Where, solved examples for calorimetry formula. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The formula q = ccal × δt is essential for calorimetry experiments using a calorimeter. The symbol c stands for the. It's not easy to measure the heat transfer directly. A physics class has been assigned the task to determine an. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or.
PPT Calorimetry PowerPoint Presentation ID6655927
Formula For Calorimeter In Physics Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Where, solved examples for calorimetry formula. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. The formula q = ccal × δt is essential for calorimetry experiments using a calorimeter. The symbol c stands for the. Qsub = m × c × δt. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. A physics class has been assigned the task to determine an. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. It's not easy to measure the heat transfer directly.
From www.tessshebaylo.com
Equation For Energy Transferred Specific Heat Capacity Tessshebaylo Formula For Calorimeter In Physics Qsub = m × c × δt. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. It's not easy to. Formula For Calorimeter In Physics.
From www.animalia-life.club
Calorimeter Diagram Formula For Calorimeter In Physics A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. Qsub = m × c × δt. The formula q = ccal × δt is essential for calorimetry experiments using a calorimeter. The symbol c stands for the. In chemistry and thermodynamics, calorimetry (from. Formula For Calorimeter In Physics.
From www.thoughtco.com
Calorimeter Definition in Chemistry Formula For Calorimeter In Physics The formula q = ccal × δt is essential for calorimetry experiments using a calorimeter. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. A container that prevents heat transfer in or out is called a calorimeter, and the use of. Formula For Calorimeter In Physics.
From www.studypool.com
SOLUTION Physics Calorimetry all formulas and notes Studypool Formula For Calorimeter In Physics A physics class has been assigned the task to determine an. The symbol c stands for the. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements. Formula For Calorimeter In Physics.
From stock.adobe.com
Vettoriale Stock illustration of chemistry and physics, Calorimeter Formula For Calorimeter In Physics Qsub = m × c × δt. The symbol c stands for the. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. A physics class has been assigned the task to determine an. Where, solved examples for calorimetry formula. The formula q = ccal × δt is essential. Formula For Calorimeter In Physics.
From socratic.org
Question 5d3f9 + Example Formula For Calorimeter In Physics A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. The formula q = ccal × δt is essential for calorimetry experiments using a calorimeter. A physics class has been assigned the task to determine an. Where, solved examples for calorimetry formula. Qsub =. Formula For Calorimeter In Physics.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Formula For Calorimeter In Physics It's not easy to measure the heat transfer directly. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Where, solved examples for calorimetry formula. A physics class has been assigned the task to determine an. Qsub = m × c × δt. A container that prevents heat transfer. Formula For Calorimeter In Physics.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to Formula For Calorimeter In Physics Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Qsub = m × c × δt. The symbol c stands for the. The formula q = ccal × δt is essential for calorimetry experiments using a calorimeter. A container that prevents. Formula For Calorimeter In Physics.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID6655927 Formula For Calorimeter In Physics A physics class has been assigned the task to determine an. The symbol c stands for the. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The formula q = ccal × δt is essential for calorimetry experiments using a calorimeter.. Formula For Calorimeter In Physics.
From www.studypool.com
SOLUTION Calorimetry formula sheet Studypool Formula For Calorimeter In Physics It's not easy to measure the heat transfer directly. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. A physics class has been assigned the task to determine an. The formula q = ccal × δt is essential for calorimetry experiments using a calorimeter. The symbol c stands. Formula For Calorimeter In Physics.
From www.pinterest.com
Image result for calorimeter (With images) Physical change, Thermal Formula For Calorimeter In Physics The formula q = ccal × δt is essential for calorimetry experiments using a calorimeter. The symbol c stands for the. A physics class has been assigned the task to determine an. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature.. Formula For Calorimeter In Physics.
From www.youtube.com
Physics 9.09a Calorimetry YouTube Formula For Calorimeter In Physics It's not easy to measure the heat transfer directly. The symbol c stands for the. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of. Formula For Calorimeter In Physics.
From www.youtube.com
Physics 9.09g Calorimetry Example 2 YouTube Formula For Calorimeter In Physics The formula q = ccal × δt is essential for calorimetry experiments using a calorimeter. Qsub = m × c × δt. Where, solved examples for calorimetry formula. A physics class has been assigned the task to determine an. It's not easy to measure the heat transfer directly. A container that prevents heat transfer in or out is called a. Formula For Calorimeter In Physics.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Formula For Calorimeter In Physics In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Where, solved examples for calorimetry formula. Qsub = m × c × δt. The symbol c stands for the. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the. Formula For Calorimeter In Physics.
From bettyelevisxo.blob.core.windows.net
Fundamentals Of Calorimetry Lab Formula For Calorimeter In Physics Qsub = m × c × δt. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. The symbol c stands for the. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance,. Formula For Calorimeter In Physics.
From haipernews.com
How To Calculate Heat Capacity From Calorimeter Haiper Formula For Calorimeter In Physics In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. A physics class has been assigned the task to determine an. The formula q = ccal × δt is essential for calorimetry experiments using a calorimeter. It's not easy to measure the heat transfer directly. The symbol c stands. Formula For Calorimeter In Physics.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo Formula For Calorimeter In Physics It's not easy to measure the heat transfer directly. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The formula. Formula For Calorimeter In Physics.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID5577734 Formula For Calorimeter In Physics The formula q = ccal × δt is essential for calorimetry experiments using a calorimeter. Qsub = m × c × δt. The symbol c stands for the. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Where, solved examples for calorimetry formula. A physics class has been. Formula For Calorimeter In Physics.